Mangostanin Derivatives as Novel and Orally Active Phosphodiesterase 4 Inhibitors for the Treatment of Idiopathic Pulmonary Fibrosis with Improved Safety.
Huang, Y.Y., Deng, J., Tian, Y.J., Liang, J., Xie, X., Huang, Y., Zhu, J., Zhu, Z., Zhou, Q., He, X., Luo, H.B.(2021) J Med Chem 64: 13736-13751
- PubMed: 34520193
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01085
- Primary Citation of Related Structures:
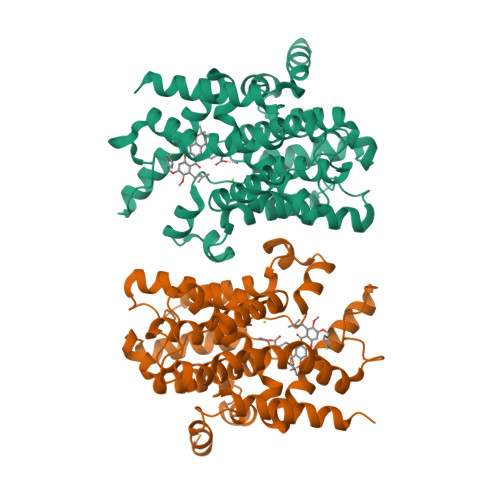
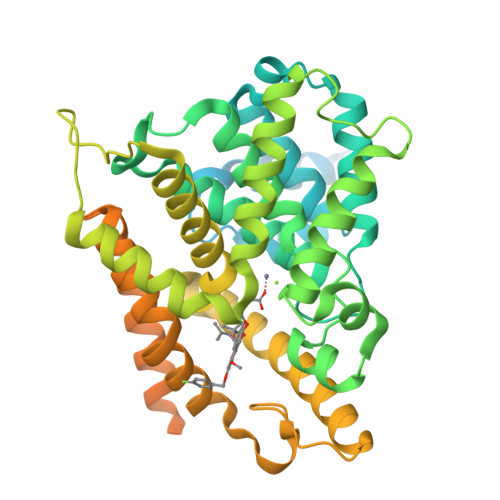
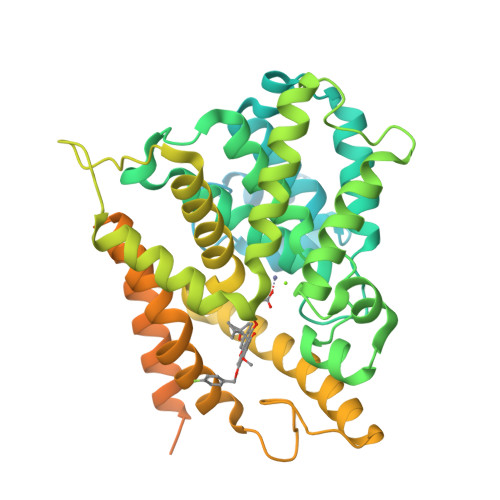
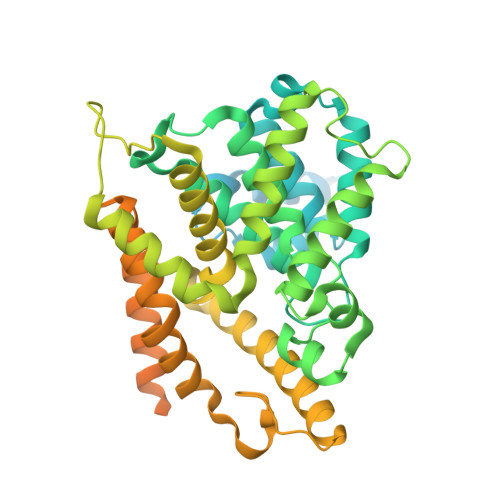
7F2K, 7F2L, 7F2M - PubMed Abstract:
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease, and its incidence rate is rapidly rising. However, effective therapies for the treatment of IPF are still lacking. Phosphodiesterase 4 (PDE4) inhibitors were reported to be potential anti-fibrotic agents, but their clinical use was hampered by side effects like emesis and nausea. Herein, structure-based hit-to-lead optimizations of natural mangostanin resulted in the novel and orally active PDE4 inhibitor 18a with potent inhibitory affinity (IC 50 = 4.2 nM), favorable physico-chemical properties, and a different binding pattern from roflumilast. Emetic activity tests on dogs demonstrated that 18a cannot cause emesis even at an oral dose of 10 mg/kg, whereas rolipram had severe emetic effects at an oral dose of 1 mg/kg. Finally, the oral administration of 18a (10 mg/kg) exhibited comparable anti-pulmonary fibrosis effects with pirfenidone (150 mg/kg) in a bleomycin-induced IPF rat model, indicating its potential as a novel anti-IPF agent with improved safety.
Organizational Affiliation:
Key Laboratory of Tropical Biological Resources of Ministry of Education, School of Pharmaceutical Sciences, Hainan University, Haikou 570228, China.